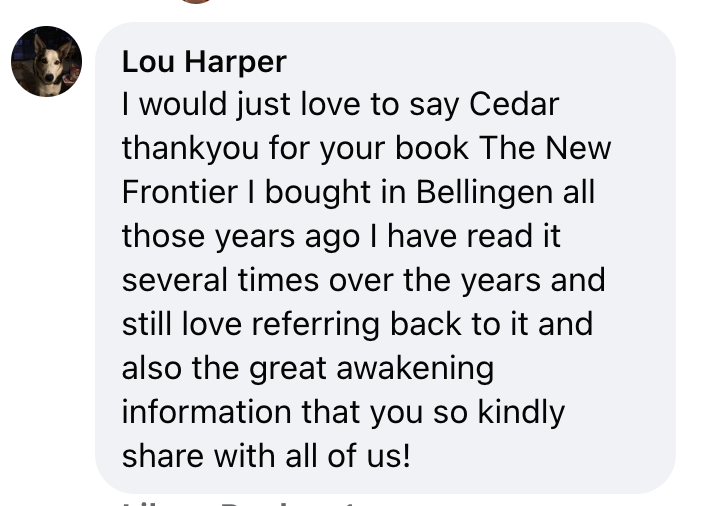
| | Heartfelt gratitude to the Albanese Labor Government for the long-awaited apology to all the people and their families who have suffered and are still suffering from the effects of the drug thalidomide. Those affected have to endure unimaginable deprivation, harassment and pain through no fault of their own. |
On 1 October 1957, Chemie Gruenenthal launched thalidomide on the market and began marketing it under the trade name Contergan. In the UK, thalidomide was authorised in 1958 by the Distillers Company (Biochemicals) Ltd, a subsidiary of Distillers Co Ltd (now part of Diageo plc), under the brand name Distaval.
In the USA, Chemie Gruenenthal approached Smith, Kline & French (SKF), now GlaxoSmithKline, with a request to market and distribute the drug in North America. However, the US Food and Drug Administration (FDA) refused to approve thalidomide for marketing and distribution.
By the mid-1950s, 14 pharmaceutical companies were marketing thalidomide in 46 countries under at least 37 different trade names. In November 1961, however, thalidomide was withdrawn from the market due to increasing evidence of its teratogenic (birth defect-causing) effects.
To this day, unethical and greedy pharmaceutical companies ignore the potentially devastating consequences of inadequately tested or known defective drugs and vaccines and expose them to the public who accept them in good faith.